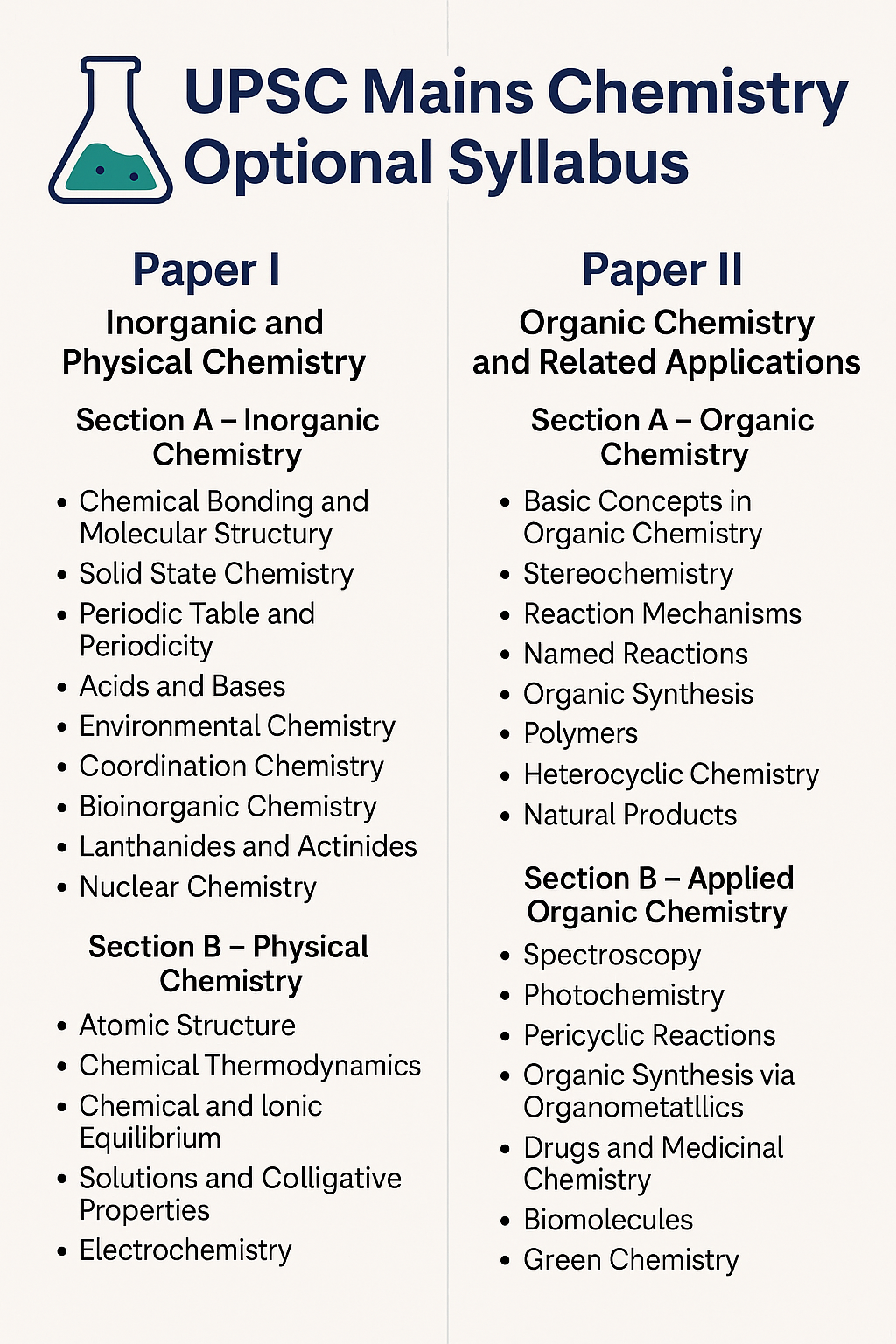
UPSC Mains Chemistry Optional Syllabus (Paper I & Paper II) – Detailed Breakdown
The Chemistry optional is a highly scientific and logical subject in the UPSC Civil Services Mains Examination. Candidates with a background in Chemistry, Pharmacy, Chemical Engineering, or related fields often opt for this subject due to its scoring potential and clear-cut nature.
The syllabus is divided into Paper I and Paper II, each carrying 250 marks. Here’s a comprehensive, topic-wise descriptive breakdown of the syllabus.
Join WhatsApp community for Free Notifications, Updates, Study Material, Mock Tests, Internship Updates, and Current Affairs - CLICK HERE TO JOIN
Paper I: Inorganic and Physical Chemistry
Section A – Inorganic Chemistry
Chemical Bonding and Molecular Structure
- Ionic Bonding: Factors affecting ionic bond strength, Born-Haber cycle, lattice energy.
- Covalent Bonding: Valence bond theory, molecular orbital theory (homonuclear and heteronuclear diatomic molecules), hybridization, VSEPR theory.
- Bond Parameters: Bond order, bond length, bond angle, and bond enthalpy.
- Coordinate Bonding & Metallic Bonding: Explanation through orbital overlap and band theory.
Solid State Chemistry
- Crystal Systems & Lattices: Unit cell, Bravais lattices, Miller indices, and crystal defects.
- X-ray Diffraction: Bragg’s law, determination of crystal structure.
- Types of Solids: Ionic, molecular, covalent network, and metallic solids.
- Imperfections: Point defects, dislocations, and their influence on properties.
Periodic Table and Periodicity
- Classification of Elements: Modern periodic law, long-form periodic table.
- Trends: Ionization energy, electron affinity, atomic/ionic radii, electronegativity.
Acids and Bases
- Arrhenius, Brønsted-Lowry, and Lewis concepts.
- HSAB principle (Hard and Soft Acids and Bases).
- pH calculations and acid-base equilibria in aqueous and non-aqueous solvents.
Environmental Chemistry
- Pollutants: Air, water, and soil pollution, greenhouse gases, and global warming.
- Chemical Reactions in Atmosphere: Photochemical smog, ozone depletion.
- Waste Management: Chemical treatment of industrial and nuclear waste.
Coordination Chemistry
- Werner’s Theory, Valence Bond Theory, and Crystal Field Theory.
- Isomerism: Structural and stereoisomerism in coordination compounds.
- Stability of Complexes: Thermodynamic and kinetic aspects.
- Spectral and Magnetic Properties: Electronic spectra and magnetic moments.
Bioinorganic Chemistry
- Role of metal ions in biological systems: Hemoglobin, myoglobin, and chlorophyll.
- Metalloproteins and metalloenzymes.
- Metal toxicity and metal deficiency diseases.
Lanthanides and Actinides
- Electronic configuration, oxidation states, color, and magnetic properties.
- Separation techniques and lanthanide contraction.
- Comparison of lanthanides and actinides.
Nuclear Chemistry
- Radioactivity: Types of decay, nuclear stability, and binding energy.
- Nuclear Reactions: Fission and fusion.
- Kinetics of radioactive decay and its applications in dating.
Join WhatsApp community for Free Notifications, Updates, Study Material, Mock Tests, Internship Updates, and Current Affairs - CLICK HERE TO JOIN
Section B – Physical Chemistry
Atomic Structure
- Quantum theory, wave-particle duality, Schrödinger equation, quantum numbers.
- Hydrogen atom, radial and angular wave functions.
- Periodic properties in terms of quantum numbers.
Chemical Thermodynamics
- First, second, and third laws of thermodynamics.
- Gibbs and Helmholtz free energy.
- Criteria for spontaneity, partial molar quantities, chemical potential.
Chemical and Ionic Equilibrium
- Law of mass action, Le Chatelier’s Principle.
- Ionic equilibria in aqueous solutions – buffer, solubility product, common ion effect.
Solutions and Colligative Properties
- Raoult’s Law, ideal and non-ideal solutions.
- Determination of molecular weights from colligative properties.
Electrochemistry
- Conductance, electrochemical cells, Nernst equation.
- EMF, pH meters, concentration cells.
- Electrolysis and overpotential.
Chemical Kinetics
- Order and molecularity, rate laws, and integrated rate equations.
- Arrhenius equation, activation energy.
- Catalysis – homogeneous and heterogeneous.
Surface Chemistry
- Adsorption isotherms (Langmuir and Freundlich).
- Colloids, emulsions, micelles.
- Surfactants and detergents.
Quantum Chemistry
- Postulates of quantum mechanics.
- Operators, eigenfunctions and eigenvalues.
- Particle in a box and harmonic oscillator models.
Statistical Thermodynamics
- Concepts of microstates and macrostates.
- Boltzmann distribution, partition function.
- Application to ideal gases.
To Enroll in FIRST IAS INSTITUTE - Click Here
Paper II: Organic Chemistry and Related Applications
Section A – Organic Chemistry
Basic Concepts in Organic Chemistry
- Inductive, mesomeric, hyperconjugation, and electromeric effects.
- Reactive intermediates: Carbocations, carbanions, free radicals, carbenes, nitrenes.
- Types of organic reactions: Substitution, addition, elimination, rearrangement.
Stereochemistry
- Geometrical and optical isomerism.
- Chirality, R/S and E/Z nomenclature.
- Conformational analysis of alkanes and cycloalkanes.
Reaction Mechanisms
- Mechanisms of SN1, SN2, E1, E2.
- Electrophilic and nucleophilic aromatic substitution.
- Addition to carbonyl groups, rearrangement reactions.
Named Reactions
- Aldol condensation, Cannizzaro, Perkin, Claisen, Wittig, etc.
- Detailed mechanism and synthetic utility of important named reactions.
Organic Synthesis
- Retrosynthetic analysis and synthetic strategies.
- Use of reagents like PCC, DCC, LiAlH4, NaBH4.
- Protecting groups and chemoselectivity.
Polymers
- Classification and methods of polymerization.
- Structure-property relationships.
- Biodegradable and conducting polymers.
Heterocyclic Chemistry
- Chemistry of five and six-membered heterocycles: Pyrrole, furan, thiophene, pyridine.
- Reactivity and synthesis of heterocycles.
Natural Products
- Alkaloids: Isolation, classification, and structural elucidation (e.g., nicotine, quinine).
- Terpenoids: Classification and biosynthesis.
- Vitamins and Hormones: Structural features and biological functions.
Join WhatsApp community for Free Notifications, Updates, Study Material, Mock Tests, Internship Updates, and Current Affairs - CLICK HERE TO JOIN
Section B – Applied Organic Chemistry
Spectroscopy
- UV-Vis: λmax, chromophores, auxochromes.
- IR: Functional group identification.
- NMR: Chemical shift, spin-spin coupling.
- Mass Spectrometry: Fragmentation patterns and molecular ion peaks.
Photochemistry
- Jablonski diagram, fluorescence, phosphorescence.
- Photo-induced reactions and quantum yields.
Pericyclic Reactions
- Electrocyclic, cycloaddition, and sigmatropic rearrangements.
- Woodward-Hoffmann rules.
Organic Synthesis via Organometallics
- Grignard reagents, organolithium compounds.
- Applications in carbon-carbon bond formation.
Drugs and Medicinal Chemistry
- General principles of drug action.
- Structure-activity relationship (SAR).
- Synthesis of common drugs: Aspirin, paracetamol, sulfa drugs.
Biomolecules
- Carbohydrates: Classification, reactions, and stereochemistry.
- Proteins: Structure levels, peptide synthesis.
- Nucleic Acids: DNA, RNA – structure and function.
- Lipids: Fatty acids, triglycerides, and their biological roles.
Green Chemistry
- Principles and importance.
- Green solvents, catalysts, and alternative reaction conditions.
- Atom economy and sustainable chemical practices.
To Enroll in FIRST IAS INSTITUTE - Click Here
Final Thoughts
The Chemistry Optional syllabus for UPSC Mains is comprehensive and technical, demanding a sound understanding of fundamental concepts and the ability to apply them in a descriptive and analytical manner. Aspirants should focus on:
- Clear concept-building through NCERTs and standard books (like Morrison & Boyd, JD Lee, Atkins).
- Diagrams and mechanisms wherever relevant.
- Previous year questions to understand the exam pattern.
- Regular revision and answer writing practice.

 firstiasofficial@gmail.com
firstiasofficial@gmail.com
Leave a Comment